Compliance
Our manufacturing facilities maintain a comprehensive Quality Management System, integrating
the control, monitoring and management of quality at the production source and in order to be a leading
supplier, Tritone strives for outstanding performance in all that we do. All employees are driven by the
following fundamental values without compromise:
• Create an enjoyable, positive work environment that helps our employees grow, motivates
them to take responsibility, and gives them a clear understanding that they can make a
difference and rewards them for our success.
• We listen to our customers so that we may identify their needs and provide them with the best
solution and the best value.
• We strive to provide our customers with the latest technologies, the most innovative products
with the best technical support and service in the industry.
• Identify those suppliers who have the same commitment to excellence that we do and form a
true partnership to ensure each other’s long-term success.
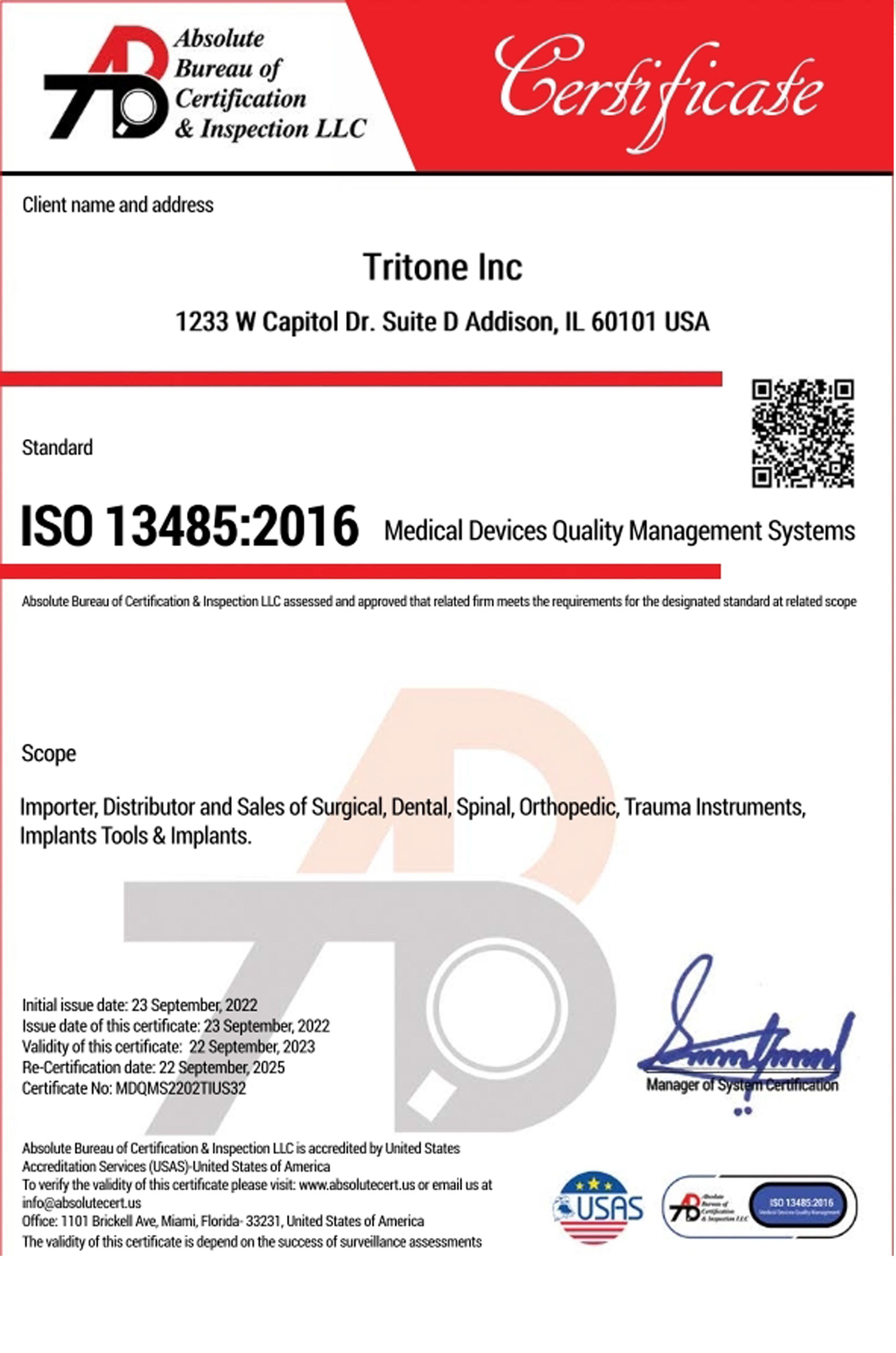
Our quality systems are based upon and in compliance with International Organization for
Standardization requirements (ISO), and where applicable, United States Food and Drug Administration
regulations (FDA).
QMS

ISO 13485:2016

ISO 9001:2015
ISO 13485:2016
QMS
Tritone Falls Under Umbrella Structure Of Stone Instruments Co
2025 Tritone . All Rights Reserved.